Aug 21, 2025
Professor of Chemistry Dr. Scott Simpson, in collaboration with researchers at the University at Buffalo, has developed a more accurate way to measure the acidity of “forever chemicals,” a crucial step in understanding how these pollutants move through the environment and affect health.
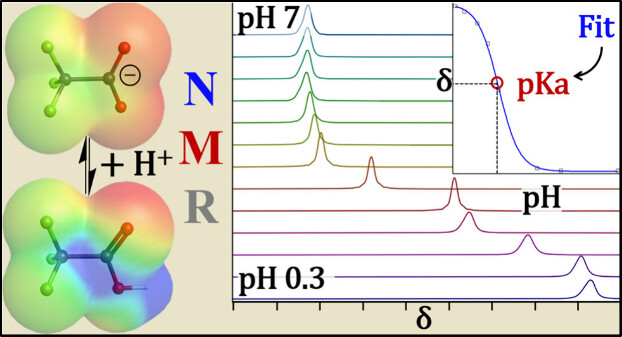 “Forever chemicals,” known scientifically as per- and polyfluoroalkyl substances (PFAS), are used in products such as nonstick pans, waterproof jackets, and firefighting foam. They’re called “forever” because they don’t easily break down, and they can build up in people, animals and water supplies.
“Forever chemicals,” known scientifically as per- and polyfluoroalkyl substances (PFAS), are used in products such as nonstick pans, waterproof jackets, and firefighting foam. They’re called “forever” because they don’t easily break down, and they can build up in people, animals and water supplies.
Using a specialized nuclear magnetic resonance (NMR) technique and high-performance computing (HPC), the researchers found that some PFAS are far more acidic than previously thought, meaning they dissolve and spread in water more easily. Others, such as certain modified PFAS, are much less acidic and may behave differently in nature.
The researchers’ work, titled “Experimental Determination of pKa for 10 PFAS, Mono-, Di-, and Trifluoroacetic Acid by 19F-NMR,” was published in the American Chemical Society journal Environmental Science & Technology Letters. The journal’s aim is to quickly publish short, timely research of an urgent or emerging nature on complex environmental phenomena while simultaneously facilitating the solution of critical environmental problems.
“Why does this matter to the average person? A chemical’s acidity, measured by its pKa, determines how it travels through soil, air and water, and how likely it is to get into drinking water or the food chain,” said Simpson. “The new results mean that many PFAS could be more mobile — and harder to contain — than earlier studies suggested. This has direct implications for how communities monitor water quality, how cleanup projects are planned, and even how future regulations are set.”
Accurate data like this helps scientists predict where these chemicals will go, how long they’ll linger, and how to remove them from the environment. For communities affected by PFAS contamination, it could mean more effective clean-up strategies and stronger safeguards for safe drinking water.